Content
Vascular Access
Click on an item to jump to that section.
- Multimedia
- Introduction
- Imaging techniques and available equipment
- What's wrong with my technique?
- Steps to success
- References
Multimedia
Click the links to open a video or interactive image demonstrating vascular access. Flash player is required to view this content.
- Radial artery: short-axis view (video)
- Radial artery: short-axis view, colour Doppler (video)
- Radial artery: long-axis view (video)
- Radial artery: long-axis view, colour Doppler (video)
- Radial artery: cannulation in-plane (interactive application)
- Internal jugular vein and carotid artery: short-axis view (video)
- Internal jugular vein and carotid artery: short-axis view, colour Doppler (video)
- Internal jugular vein and carotid artery: short- and long-axis view (video)
- Internal jugular vein and carotid artery: short- and long-axis view, colour Doppler (video)
Introduction
Central venous catheterization (CVC) and arterial catheterization are common procedures performed by anesthesiologists. Traditionally, a landmark-based approach has been utilized to obtain vascular access. However, the introduction of perioperative ultrasonography in recent years has drastically changed this practice. The use of US for vascular access has been endorsed by many practice-governing bodies.
Complications of CVC and arterial line insertion
There are many reported complications of CVC insertion. Complication rates vary secondary to differences in the definitions and methodology used in specific studies; in adults they are estimated to range from 0.5%-35%1-4.
Arterial line insertion is also not always a benign procedure. It can be complicated by hemorrhage, hematoma, vasospasm, thrombosis and injury to the adjacent nerves. Closed claim reports have documented limb ischemia with resulting amputation and other serious morbidities after attempted arterial catheterizations5. In two prospective randomized trials, real-time two-dimensional (2D) US was found to be superior to the landmark technique for arterial catheterization in adults6.
Why should I use ultrasound?
The advantages of using ultrasound to facilitate CVC and arterial line insertions include the real-time visualization of patient anatomy, and a more precise method of needle guidance compared to the landmark techniques.
Two large meta-analyses and many observational studies attest to the superiority of US guidance versus the landmark method7-8. The outcomes assessed in these studies include improved success rates, reduction in the number of attempts, a lower incidence of complications, faster procedure times and, interestingly, fewer infectious complications.
In 2001, the Agency for Healthcare Research and Quality in the United States recommended the use of 2D US-guided CVC access1. The following year, the National Institute for Clinical Excellence (NICE) in the United Kingdom issued a guidance statement on 2D US as the preferred method of IJV CVC insertion in adults and children4.
Barriers in utilizing ultrasound
In response to the NICE recommendations, opinions have been divided and implementation has been inconsistent. In the United States, 18% of cardiac anesthesiologists still do not have access to US equipment, and 67% have never or almost never used US for CVC access2.
The availability of equipment is strongly correlated with increased use. Although only 26% of clinicians use dynamic 2D US as recommended, 31% use US as a screening tool prior to the traditional landmark technique, and 40% use US as a rescue method after a landmark technique failure. Similar results are found in the UK literature2, 9.
Summary
- Even with experience, CVC cannulation using landmark techniques has a significant rate of mechanical complications. A successful first attempt is ideal. The risk of complications increases with the number of attempts.
- Two-dimensional ultrasound is superior to the landmark and Doppler techniques.
- Variability of the anatomy or thrombosis is easily identified using US, and the technique or location for vascular access can be adapted accordingly.
Imaging techniques and available equipment
- Selecting an appropriate US probe is the most important step in vascular access imaging.
- The use of a high-frequency transducer will optimize the imaging at the target vessel depth.
- A linear-array transducer is the most appropriate probe for this procedure.
- It's available in various sizes and footprints, including the small 'hockey stick' probes useful in pediatrics and for anatomy with limited access.
- Transducers have a reference marker to assist orientation to the displayed image, and an additional marker in the centre of the transducer footprint may be present.
- High-frequency (> 10 MHz) linear-array transducers (Figure 1) will provide good-resolution imaging down to a depth of approximately 4 cm, and are thus suitable for IJV cannulation, arterial catheterization and peripheral venous access.
- Lower-frequency (< 6 MHz) linear-array transducers and phased-array probes have better tissue penetration, and may be useful for cannulation of large, deep vessels such as the femoral vessels in obese patients. Their disadvantage is a lower resolution image.
- Needle-guide kits (Figure 2) are commercially available and may be useful in directing the needle to the target; however, the freehand technique is preferable once sufficient experience is gained.
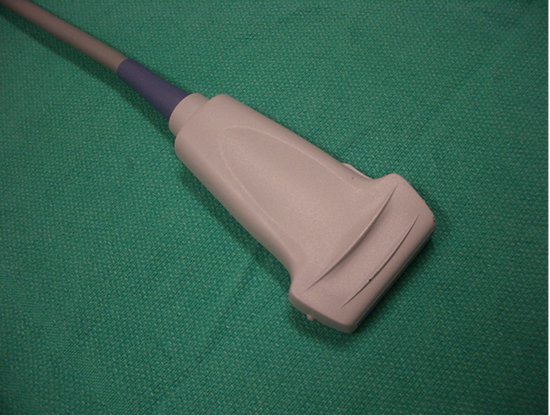
Figure 1. Linear array transducer; large footprint (38 mm).

Figure 2. Linear array transducer with needle guide attachment10.
What's wrong with my technique?
- Optimal images are generated by ensuring good skin–probe contact and the correct US machine settings, and by paying attention to ergonomics.
- Skin-probe contact is improved by holding the transducer close to the footprint, applying appropriate pressure, and employing a coupling medium such as a US transmission gel or saline.
- Veins are very compressible, and excessive pressure collapses the target vein.
- The US machine should be preset to the vascular mode, if available.
- Further fine-tuning of the displayed image is accomplished by choosing an appropriate depth of field, adjusting the near- and far-field controls, and placing the focus point at the target depth.
- Set the 'Gain' to clearly delineate the vessel walls against the lumen.
- Ensure that the displayed image is oriented correctly, and that it corresponds to the reference marker on the probe.
- Finally, position the patient and the US machine in your direct line of vision, with easy access to the target vessel and the cannulation equipment.
Steps to success
A methodical approach to locating and identifying vessels with US will minimize errors (10). Vessels should be imaged in two planes to reconstruct their three-dimensional structure, using both the out-of-plane (also called the transverse or cross-sectional) view and the in-plane (also called the longitudinal) view.
Real-time in-plane and out-of-plane methods
- Identify the orientation of the probe with respect to the ultrasound screen.
- Visualize the vessels using the transverse orientation of the probe.
- The target, in this case of vessels, should be at the centre of the screen.
- Recognize the sonoanatomy: vein compressibility, artery pulsatility, response to Valsalva, color flow Doppler and pulse wave Doppler.
- For the out-of-plane technique (Figure 3), centre the vessels on the display screen.
- Using small flicker movements, identify the needle entry site on the skin.
- Using real-time image guidance, advance the needle slowly towards the target vessel.
- For the in-plane approach (Figure 4): after Step 4, re-orient the probe to the longitudinal direction and centre the vessel on the display screen.
- Confirm the identification of the vessels by repeating Step 4.
- Repeat Steps 6 and 7.
- Check for guide wire cannulation of the correct vessel before dilating.
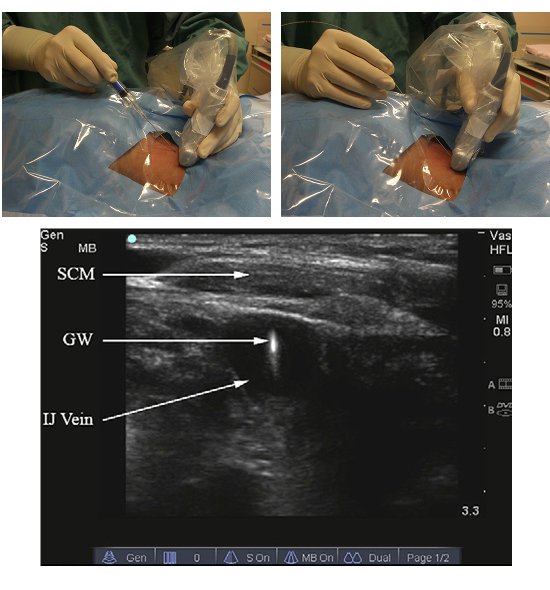
Figure 3. The real time out-of-plane method. Needling technique (top left); guide wire insertion (top right) and sonographic view (bottom).
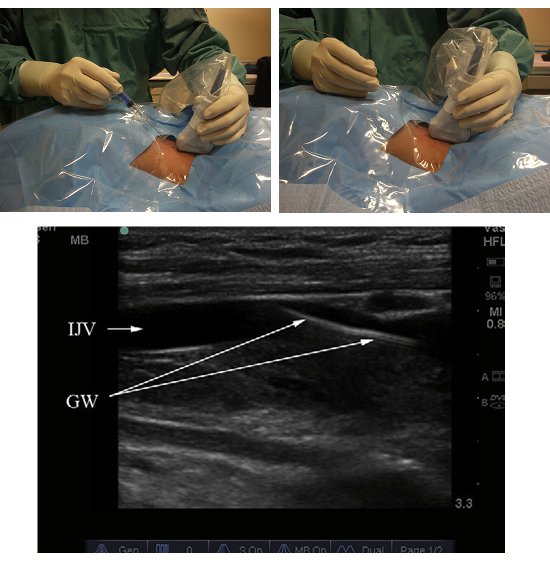
Figure 4. The real time in-plane method. Needling technique (top left); guide wire insertion (top right) and sonographic view (bottom).
Indirect US technique
- Identify the orientation of the probe with respect to the ultrasound screen.
- Visualize the vessels using the transverse orientation of the probe.
- The target, in this case of vessels, should be at the centre of the screen.
- Recognize the sonoanatomy: vein compressibility, artery pulsatility, response to Valsalva, color flow Doppler and pulse wave Doppler.
- If using the indirect US technique, measure the depth and mark the pathway of the vessel above and below the insertion point (Figure 5).
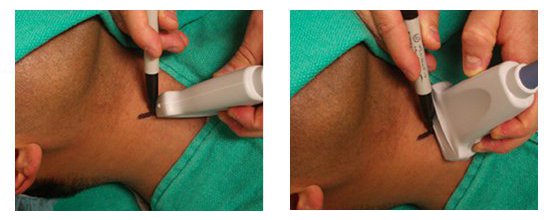
Figure 5. The indirect method. Long axis (left); short axis (right).
Ultrasound-guided radial artery catheterization
Similar to the methods described for IJV cannulation, the indirect US technique (Figure 6) can be used, or real-time 2D US guidance (Figure 7). The steps, concept and techniques remain similar.
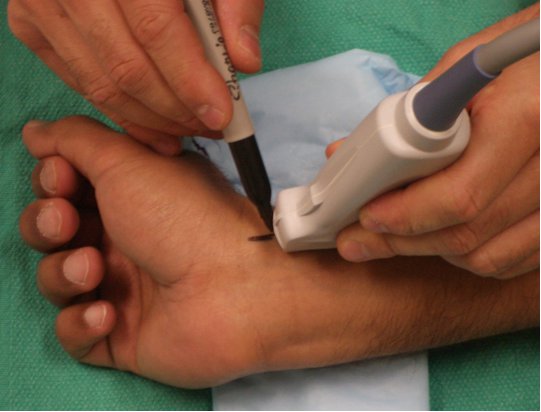
Figure 6. Indirect technique for radial artery catheterization.
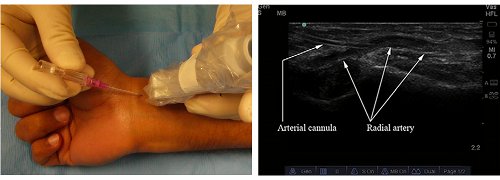
Figure 7. Real-time method of radial artery catheterization. Needle insertion (left); sonographic view (right).
References
- Rothschild JM. Ultrasound guidance of central vein catheterization. In agency for healthcare research and quality. Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment July 2001;43.
- Bailey PL, Glance LG, Eaton MP, et al. A survey of the use of ultrasound during central venous catheterization. Anesthesia and Analgesia 2007;104:491–497.
- Leung J, Duffy M, Finckh A. Real-time ultrasonographically-guided internal jugular vein catheterization in the emergency department increases success rates and reduces complications: a randomized, prospective study. Annals of Emergency Medicine 2006;48:540–547.
- National Institute for Clinical Excellence. Guidance on the use of ultrasound locating devices for placing central venous catheters. Technology Appraisal Guidance. Number 49. September 2002.
- Levin PD, Sheinin O, Gozal Y. Use of ultrasound guidance in the insertion of radial artery catheters. Critical Care Medicine 2003; 31: 481–484.
- Shiver S, Blaivas M, Lyon M. A prospective comparison of ultrasound-guided and blindly-placed radial arterial catheters. Academic Emergency Medicine 2006;13:1275–1279.
- Bowdle TA. Central line complications from the ASA closed claims project. ASA Newsletter 1996;60:22–25. Domino KB, Bowdle TA, Posner KL, et al. Injuries and liability related to central vascular catheters. A closed claims analysis. Anesthesiology 2004;100:411–418.
- Scott DH. It's NICE to see in the dark. British Journal of Anaesthesia 2003;90:269–272.
- Randolph AG, Cook DJ, Gonzales CA, et al. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Critical Care Medicine 1996;24:2053–2058.
- Kumar A, Chuan A. Ultrasound guided vascular access: efficacy and safety. Best Pract Res Clin Anaesthesiol. 2009 Sep;23(3):299-311.

